
IEC 报告、X 光机、MRI、CT、超声仪等大型医疗器械的产品说明书及注册材料,辐射学、核医学、实验室及临床各科诊断及检测设备,血液生化检测、ELISA(酶联免疫吸附实验)试剂盒、基因芯片、蛋白芯片技术等。
药理学、毒理学、生药学、药物化学、药物分析学、药剂学、制剂处方及工艺、原辅料来源及质量标准、药品检测报告、生物制药、新药急性毒性、慢性毒性试验、新药临床试验、新药报批资料全套的整理和翻译,研究者手册等。
食品、饮料、化妆品、护肤品、以及保健品等。
兽医学、畜牧学、宠物驯养、兽用医疗器械、兽用药物、猫粮、狗粮等资料。
生命科学领域国际学术会议、研讨会演讲 ppt 制作和翻译、视频影像资料,大会日程及纪录翻译等。
医学科普公共信息类;病历及住院记录;医药网站、宣传资料等;医学网站及数据库本地化;医学软件使用手册及界面汉化等。
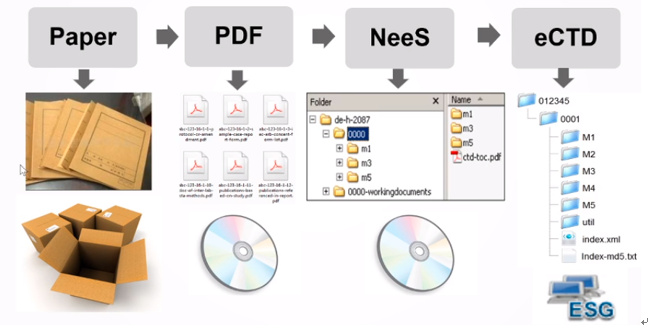
eCTD 是英文 Electronic Common Technical Document 的简称,eCTD 由 CTD 发展而来。随着医药研发的演进,注册文件递交的方式,总共经历了四种变化。
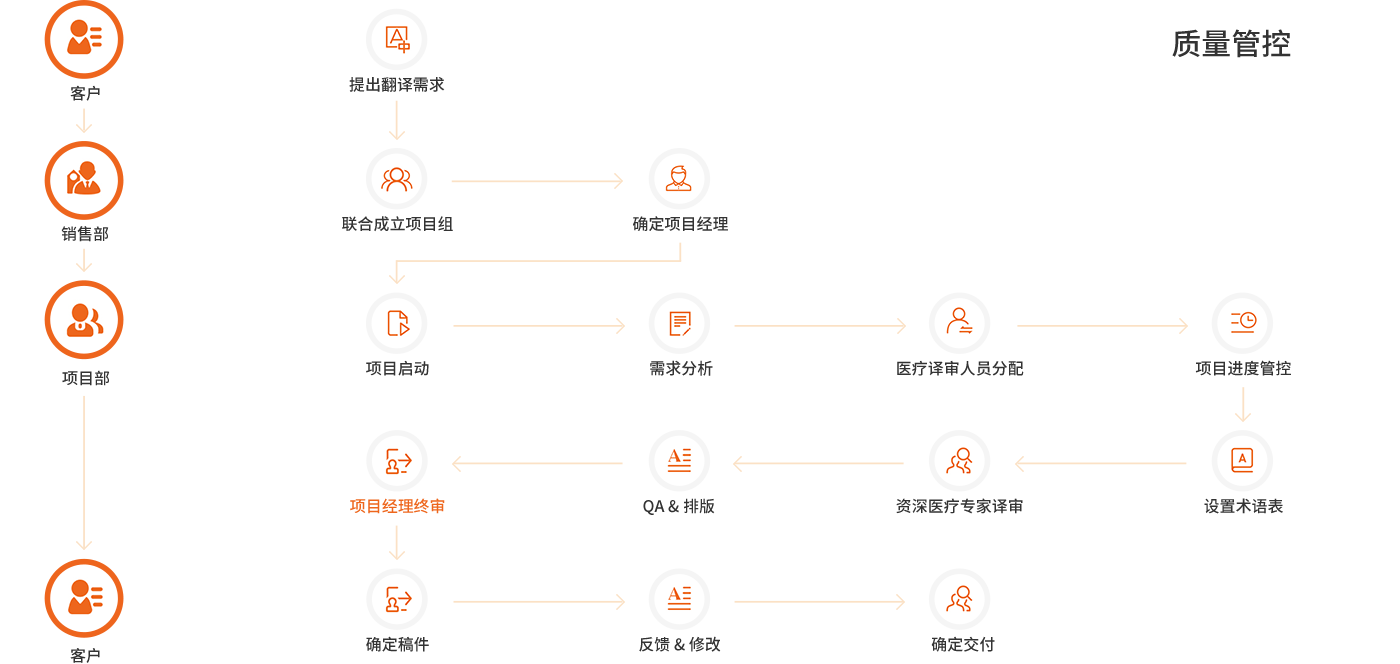
我们的翻译团队由 5 年以上从业经验的译者与审校组成,专业背景扎实、术语敏感度高,译文专业准确、风格一致。
对于行业专业术语具有很强的敏感性。为客户提供的翻译成果专业、准确。
· 通过 ISO 9001、ISO 17100、ISO/IEC 27001 认证
· 精通医药与医疗器械等细分领域
· 熟悉多国法规要求,译文准确、及时、合规
· 快速响应、准时交付,保障产品注册与上市进度